MS Patient Survey: Why Pharma Should be Providing Treatment Info & Education
A survey taken by a sample of the 7,000+ MyTherapy users living with MS reveals that most want more information regarding alternative multiple sclerosis treatments. Read why pharma should deliver it and how they can do so

- MyTherapy users living with multiple sclerosis tell us they want more information about alternative MS treatments
- Pharma has a role in effectively delivering MS patient education. Publishing information is no longer enough
- Regulatory compliance is critical for direct-to-patient initiatives – but it doesn’t need to be an obstacle
With over 7,000 people living with multiple sclerosis using MyTherapy to manage their treatment, we can gain valuable insight that helps both us and our partners deliver better support and education. Read on to find out how our latest survey suggests that users are keen to receive more information about the latest MS treatments and why pharma could and should be playing a bigger role in providing it.
MyTherapy Survey: What Did MS Users Say?
In June 2021 we ran a survey among a sample of MyTherapy users living with MS. Among other questions, we asked: “Are you satisfied with your current MS medications?”. The good news: In the survey covering patients on treatments such as Tecfidera (Dimethyl Fumarate, Biogen), Aubagio (Teriflunomide, Sanofi), and Copaxone (Glatiramer Acetate, Teva), only a little less than 20% of patients are not sure if their treatment works for them.
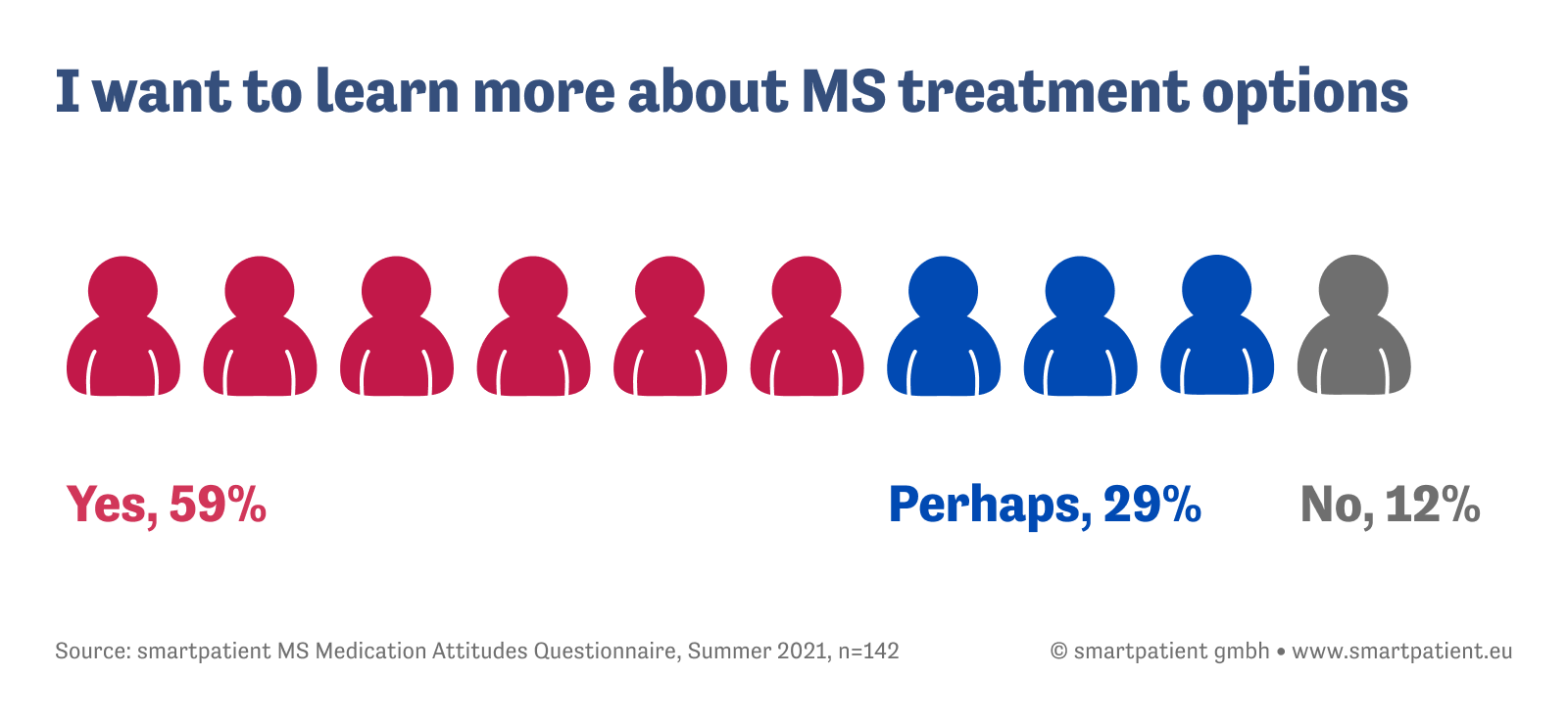
At the same time, the survey uncovers a significant patient need: Nearly 90% of respondents said they would (59%) or might (29%) be interested in learning more about alternative treatments. Given the research and innovation taking place in the world of MS treatment, such as result is hardly surprising. The question for healthcare and pharma alike, though, is how to better inform patients on the latest research and innovative medicines in the field of multiple sclerosis.
Why Pharma Should Deliver MS Patient Information & Education
In a previous article on this blog, a couple both living with MS said that “pharmaceutical companies generally aren’t the first places we turn” for information. Another said he is unaware of any direct lines of communication between pharma and patients. Yet, as the manufacturer of the drugs, pharmaceutical companies are the source of first-hand information and, therefore, best placed to ensure it is safe and reliable.
Doing so is easier than ever.
This survey was completed by only a portion of the thousands of MyTherapy users living with MS. This userbase is testament to our belief that people living with MS do not consider themselves ‘MS patients’ but ‘users’: Each of them has health needs relating to other treatments and lifestyles and wants to live her best life possible. To this end, these users clearly desire information relating to MS. This is why we are launching MyTherapy for MS, which allows users to access a layer of content and functionalities relating specifically to multiple sclerosis, on top of the regular features that have made MyTherapy such a widely used health management app.
For pharma, we can take it a step further and reach patients on specific treatments. This triple-layer offering means we can support users’ daily needs, along with disease- and drug-specific modules, all within one app that patients use multiple times per day and with millions of users around the world.
It creates the opportunity for pharma support and engage patients in a quality-controlled, compliant environment, including the provision of the so-sought-after information, e.g. around treatment options. Not only do users benefit from a more seamless experience, they also benefit from reliable information that comes straight from the source while using an app that supports any treatment and comorbidity.
Education and Information Need Not Be Promotional
One of the comments we hear regularly when discussing with our pharma partners is that providing educational content regarding treatment options could be considered promotional, thus contravening regulations around the globe. However, this is not the case – or, rather, it does not need to be the case. Our layered platform design allows us to strictly segregate disease support from product-related information. And while the former should be broadly available to support as many patients as possible, product-related contents are only accessible to patients with the appropriate prescription.
If you would like to discuss how you and patients on your treatment can benefit from MyTherapy, don’t hesitate to get in touch.